EPICARDIAL ADIPOSE TISSUE GENE EXPRESSION CHANGES IN PATIENTS WITH ADVANCED HEART FAILURE: THE IMPACT OF CARDIAC CACHEXIA
Introduction: Epicardial adipose tissue (EAT) may serve as a transducer of metabolic influences on the heart. The determinants of EAT gene expression in advanced heart failure (HF) are not known.
Methods: We examined EAT from 54 HF patients undergoing heart TX (age: 49±7y, 83% males, 37% ischemic, BMI 25±4.0 kg.m-2) and 20 controls (HF-free organ donors) of similar BMI, age and gender. EAT from anterior interventricular area was harvested at TX. After RNA isolation, gene expression analysis (qPCR) was performed (LightCycler, Roche) and normalized to the mean of GAPDH and EF-1A genes.
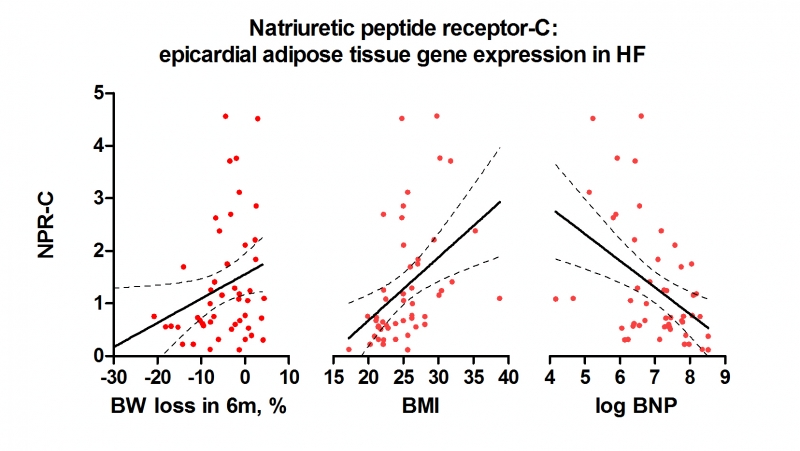
Results: Compared to controls, EAT of HF patients displayed profound upregulation of metabolic transcription factors PGC1a and PPARa, upregulation of adipokines (leptin, adiponectin) and enhanced prolipolytic signalling (natriuretic peptide receptors A and C, hormone sensitive lipase, perilipin, ATGL) that correlated (p˂0.05) with neurohumoral activation (log-BNP). PRDM16, marker of adipocyte browning, was not different between HF and controls.
Enhanced expression of lipolytic genes was coupled with enhanced lipogenesis (FAS, ACSL1) and TG synthesis (DGAT1, 2). From studied clinical factors (DM, age, HF etiology, BMI) within HF, EAT was most strongly influenced by cardiac cachexia (body weight-BW loss ≥5% in 6m, present in 46%). Cachectic HF had reduced expression of NP-C receptor (p˂0.001). NPR-C expression in EAT of HF subjects correlated with BMI and log-BNP. BW loss (%) correlated (p˂0.05) with PGC-1a (r: -0.27), PPARa (r:-0.32), NPR-C (r: 0.29) and leptin (r: 0.38).
Conclusions: EAT in advanced HF is characterized by enhanced lipolysis and compensatory increase in lipogenesis. HF-related cachexia affects EAT transcription profile, is associated with loss of clearance receptor for natriuretic peptides (NPR-C) leading to more profound EAT metabolic activation.